Consider a sample of oxygen at 300 K. Find the average time taken by a molecule of travel a distance equal to the diameter of the earth.
In kinetic theory of ideal gas, the average energy is given by
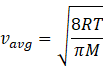
Where R=gas constant=8.31Jmol-1K-1
T=temperature of gas
M=molar mass of gas
Given
Temperature T=300K
Molar mass of oxygen=32amu=32g/mol=32![]() 10-3 kg/mol
10-3 kg/mol
Therefore
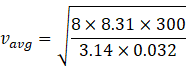
![]()
We know that
![]()
Distance here is given as diameter of earth
Now radius of earth = 6400km=6400000m
Diameter=2![]() radius
radius
So, diameter of earth =2![]() 6400000m
6400000m
So, time taken
![]()
1 hour=60![]() 60 seconds=3600seconds
60 seconds=3600seconds
So, 28747.83s =![]() h = 7.98h
h = 7.98h![]() 8h
8h
So, average time taken by oxygen molecule to travel a distance
equal to the diameter of the earth is 7.98h![]() 8h.
8h.
1