Find the average magnitude of linear momentum of a helium molecule in a sample of helium gas at 0°C. Mass of a helium molecule = 6.64 × 10–27 kg and Boltzmann constant = 1.38 × 10–23 J K–1.
In kinetic theory of ideal gas, the average energy is given by

Where R=gas constant=8.31Jmol-1K-1
T-temperature of gas
M=molar mass of gas
Given
Temperature T=0![]()
T(K)=T (![]() )+273.15
)+273.15
T=T(K)=0+273.15=273.15K
Mass of helium molecule m =6.64 × 10–27 kg
We know that,
Gas constant R=kBNA
Where kB= Boltzmann constant = 1.38 × 10–23 J K–1.
And NA=Avogadro number=6.023![]() 10-23 mol-1
10-23 mol-1

Molar mass of gas molecule M= Avogadro number![]() mass of gas molecule
mass of gas molecule
M=NA![]() m
m ![]()
So average velocity becomes
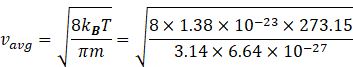
![]()
We know that
Momentum=mass![]() velocity
velocity
Momentum=6.64 × 10–27![]() 1202.31=8
1202.31=8![]() kgms-1
kgms-1
![]() Average magnitude of momentum of helium at 0
Average magnitude of momentum of helium at 0![]() is 8
is 8![]() kgms-1.
kgms-1.