Estimate the number of collisions per second suffered by a molecule in a sample of hydrogen at STP. The mean free path (average distance covered by a molecule between successive collisions) = 1.38 × 10–5 cm.
Number of collision per second means frequency of collision.
In kinetic theory of ideal gas, the average energy is given by
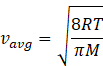
Where R=gas constant=8.31Jmol-1K-1
T-temperature of gas
M=molar mass of gas
Molar mass of hydrogen =2 amu=2![]() kg/mol
kg/mol

![]()
Given
Distance between successive collision ![]() 1.38 × 10–5 cm
1.38 × 10–5 cm
![]() 1.38
1.38![]() 10-8m
10-8m
Time between two collisions
![]()
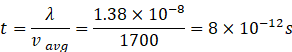
Frequency of collision =![]()
![]() Number of collision per second means frequency of collision which is equal to 1.23
Number of collision per second means frequency of collision which is equal to 1.23![]() 1011.
1011.
1