A vessel of volume V0 contains an ideal gas at pressure p0 and temperature T. Gas is continuously pumped out of this vessel at a constant volume-rate dV/dt = r keeping the temperature constant. The pressure of the gas being taken out equals the pressure inside the vessel. Find (a) the pressure of the gas as a function of time, (b) the time taken before half the original gas is pumped out.
Let P be the pressure and n be the number of moles of gas inside the vessel at any given time.
As mentioned in question, pressure is decreasing continuously. So, suppose a small amount of gas ‘dn’ moles are pumped out and the decrease in pressure is ‘dP’.
So, pressure of remaining gas =P-dP
Number of moles of remaining gas =n-dn
Given
The volume of gas =Vo
Temperature of gas=T
We know that ideal gas equation
PV=nRT
Where V= volume of gas
R=gas constant =8.3JK-1mol-1
T=temperature
n=number of moles of gas
P=pressure of gas.
So, applying ideal gas equation for the remaining gas
(P-dP) Vo=(n-dn) RT
P![]() Vo-dP
Vo-dP![]() Vo=n
Vo=n![]() RT-dn
RT-dn![]() RT …… (1)
RT …… (1)
Applying ideal gas equation, before gas was taken out
PVo = nRT ……. (2)
Using equation (2) in (1) we get
n![]() RT - Vo
RT - Vo![]() dP = n
dP = n![]() RT - dn
RT - dn![]() RT
RT
Vo![]() dP=dn
dP=dn![]() RT …… (3)
RT …… (3)
According to question, pressure of gas being taken out is equal to inner pressure of gas always. So inner pressure is equal to P-dP
Let the volume of gas taken out dV.
Applying ideal gas equation to gas pumped out
(P-dP) dV=dn![]() RT
RT
PdV=dn![]() RT …… (4)
RT …… (4)
Where we have ignored dPdV as it is very small and can be neglected.
From equation (3) and (4)
Vo![]() dP=P
dP=P![]() dV
dV
![]()
Given ![]() . Since volume is decreasing so rate should be negative.
. Since volume is decreasing so rate should be negative.
Putting this value of dV in equation (5)
![]()
(a) Integrating equation (6) from Po to P and t=0 to t
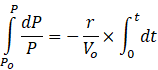
![]()
Where we have used the formula
![]()
And
![]()
![]()
![]()
Taking exponential on both sides,
![]()
![]() The pressure of the gas as a function of time is given as
The pressure of the gas as a function of time is given as ![]()
(b) In second part the final pressure becomes half of the initial pressure
![]()
Putting this value of P in equation (7)
![]()
![]()
![]()
Taking natural logarithm on both side
![]()
![]()
![]() The time taken before half the original gas is pumped out is
The time taken before half the original gas is pumped out is ![]()