Using figure of the text, find the boiling point of methyl alcohol at 1 atm (760 mm of mercury) and at 0.5 atm.
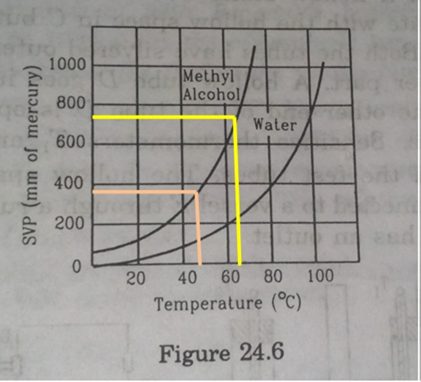
Boiling point of methyl alcohol at 1atm is 65![]() and at 0.5 atm is 48
and at 0.5 atm is 48![]() .
.
Explanation
To find the boiling point from above figure, we must look for the temperature on x-axis corresponding to pressures given in question.
So, for first pressure i.e. 1atm=760mm of Hg, the corresponding temperature on x- axis is 65![]() . This shown by yellow line in figure.
. This shown by yellow line in figure.
For the second pressure i.e. 0.5 atm
1atm=760mm of Hg
0.5atm =760![]() 0.5 mm of Hg= 375mm of Hg
0.5 mm of Hg= 375mm of Hg
For 375mm of Hg the corresponding temperature on x-axis is 48![]() . This is shown by orange line in the figure.
. This is shown by orange line in the figure.
1