Consider two processes on a system as shown in the figure.
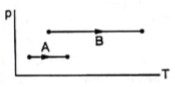
The volumes in the initial states are the same in the two processes and the volumes in the final states are also the same. Let ΔW1 and ΔW2 be the work done by the system in the processes A and B respectively.
Given ![]() V1=
V1=![]() V2
V2
We know that,
Work done = force ×displacement
![]()
Volume = area ×displacement
Therefore,
Work done=pressure ×volume
Let change in the volume of system = ΔV = V2-V1
Pressure =P
Thus, work done by the system W
ΔW=PΔV
For process A ΔW1=P1ΔV1
For process B ΔW2=P2ΔV2
Since ΔV1=ΔV2 we can write,
![]()
![]()
P1 < P2 (from graph)
Therefore ΔW1 < ΔW2.
1