Match the items of Column I with the items of Column II and assign the correct code :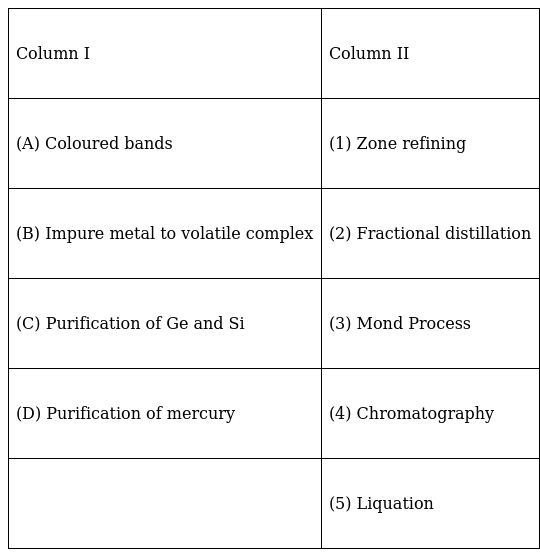
Code :
A(4) In column chromatography different compounds get adsorbed at different levels on the column. This gives rise to coloured bands which represent compounds after they get separated.
B(3) Mond’s process is a vapour phase refining technique where an impure metal like Ni forms a volatile complex with carbon monoxide giving rise to the complex Ni(CO)4. This complex on decomposition gives rise to the pure Ni metal.
C(1) Germanium and Silicon are semiconductors. They are purified using zone refining method where a molten zone passes over the solid rod. The principle behind this method is that impurities are more soluble in the molten metal than in the solid metal. The pure metal stays behind while the impurities move along with the rod to an adjacent molten zone.
D(2) Mercury has a low boiling point so it can be refined using fractional distillation. Mercury with low boiling point distilles out first and is collected as pure mercury distillate in the receiving flask while the impurities are left behind in the boiling flask.