Match the items of Column I with items of Column II and assign the correct code :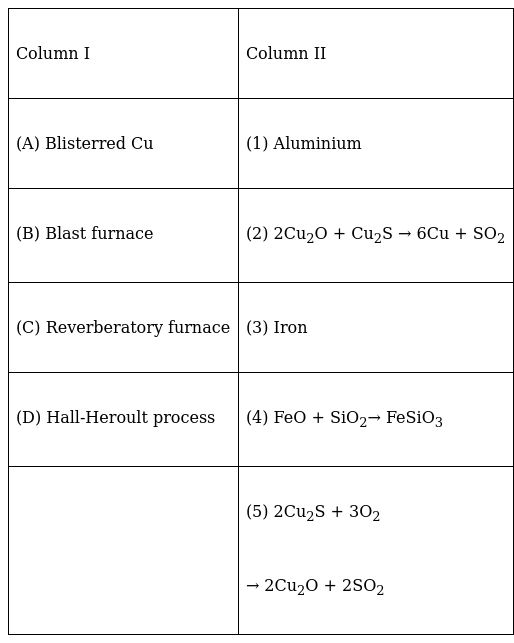
Code :
A (2) 2Cu2O + Cu2S → 6Cu + SO2
The copper produced is called Blister Copper because the evolution of SO2 gives the metal a blister appearance
B(3) A Blast furnace is used to reduce iron oxides to iron in different temperature ranges.
C(4) FeO + SiO2→ FeSiO3
Roasting of sulphide ores is done in a reverberatory furnace. The sulphide ores are converted to oxides. If there is any iron present, it is mixed with silica and forms a slag which can be easily removed.
D(1) Hall-Heroult process is used in the extraction of Aluminium
1