Match the items given in Column I with the items given in Column II.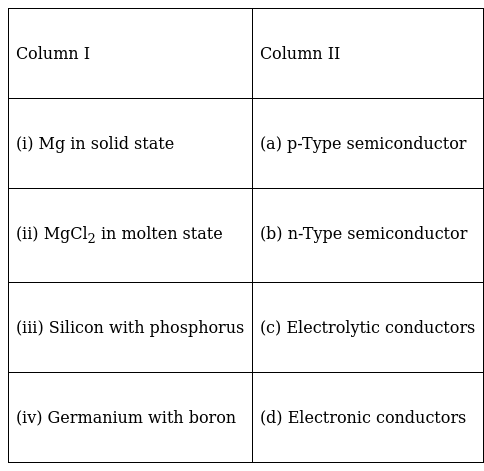
(i)-d , (ii)-c , (iii)-b , (iv)-a
(i) Mg in solid state shows electronic conductivity due to presence of free electrons, hence it is known as electronic conductors.
(ii)MgCl2 shows electrolytic conductivity in molten state due to presence of electrolytes (Mg2+ and Cl-), thus shows electrolytic conductivity.
(iii) When silicon is doped with phosphorus which contains 5 electrons. 4 electrons are used in bonds and 1 electron left delocalized and thus shows conductivity in electric field. Since the conductivity is due to electron which is negatively charged it is called N type semiconductor.
(iv) When germanium is doped with boron which contains 3 electrons, it generates one hole due to lack of electron. This hole acts as a positive charge and helps in conductivity when placed in electric field. Since the conductivity is due to positive charge it is called P type semiconductors.