Match the reactions given in Column I with the suitable reagents given in Column II.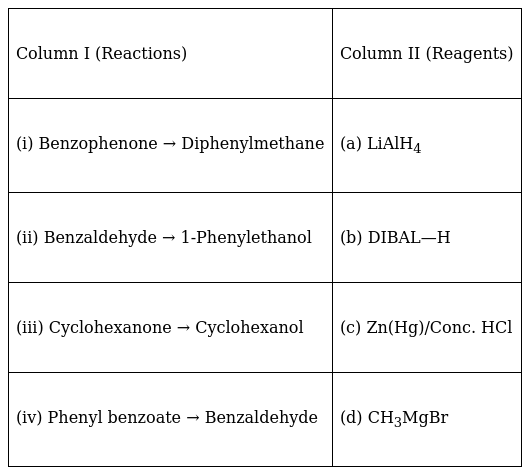
(i) Benzophenone → Diphenylmethane - (c) Zn(Hg)/Conc. HCl
(ii) Benzaldehyde → 1-Phenylethanol - (d) CH3MgBr
(iii) Cyclohexanone → Cyclohexanol - (a) LiAlH4
(iv) Phenyl benzoate → Benzaldehyde - (b) DIBAL—H
EXPLAINATION:
(i) Benzophenone is converted to Diphenylmethane by using Zn(Hg)/Conc. HCl. This is called Clemmensen reaction.

(ii) Benzaldehyde is converted to 1-Phenylethanol by using CH3MgBr. This conversion basically uses grignard reagent followed by hydrolysis.
![]() Benzaldehyde + CH3MgBr + H3O+
Benzaldehyde + CH3MgBr + H3O+
1-Phenylethanol
(iii) Cyclohexanone can be converted to cyclohexanol by a reducing agent like LiAlH4.
LiAlH4 is a strong reducing agent.
(iv) Phenyl benzoate can be converted to Benzaldehyde by reducing agent DIBAL—H.