Which of the following compounds is the weakest Brönsted base?
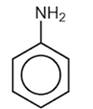
(i)
(ii)
(iii)
(iv)
• According to Brönsted (and Lowry) concept, basicity depends (i) on the tendency of a substance to accept a proton and (ii) the stability of conjugate acid(like complementary partner of the base) of the corresponding base.
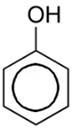
• Hence, in case of phenol  After losing H+ phenol produces least stable conjugate acid(conjugate strong acid – weak base pair, where weak base is phenol) among the given compounds. Oxygen is more electronegative than N so, O- H bond is more polar and it has a very highly acidic character. Since phenol is more acidic than that of alcohol, therefore phenol has the least tendency to accept a proton and hence it is weak Brönsted base.
After losing H+ phenol produces least stable conjugate acid(conjugate strong acid – weak base pair, where weak base is phenol) among the given compounds. Oxygen is more electronegative than N so, O- H bond is more polar and it has a very highly acidic character. Since phenol is more acidic than that of alcohol, therefore phenol has the least tendency to accept a proton and hence it is weak Brönsted base.
• Moreover, the conjugate base of phenol is resonance stabilised (4 resonating structures), which means phenol is a stronger acid rather than being a base (conjugate strong acid(SA) - weak base pair,(WB) where the SA is phenol and WB is the phenolate ion. )
 .
.
• Among the other 3 options (i)  (ii)
(ii)![]() and (iv)
and (iv) all of these are stronger base than phenol.
all of these are stronger base than phenol.
 aniline - has less electro-negative N atom, therefore has a less polar bond and a tendency to accept proton or (more precisely to donate electrons : Lewis concept) though it is a weak base but definitely a stronger one(base) than phenol and also it produces a more stable conjugate acid after accepting proton.
aniline - has less electro-negative N atom, therefore has a less polar bond and a tendency to accept proton or (more precisely to donate electrons : Lewis concept) though it is a weak base but definitely a stronger one(base) than phenol and also it produces a more stable conjugate acid after accepting proton.
• 

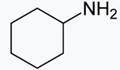
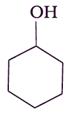
• In case of (ii)![]() and (iv)
and (iv) both are stronger base than phenol, as there is no conjugation possible for the N and O atoms attached to a cyclohexyl ring as the electrons pairs are more available for donation or proton acceptance.
both are stronger base than phenol, as there is no conjugation possible for the N and O atoms attached to a cyclohexyl ring as the electrons pairs are more available for donation or proton acceptance.