Match the following: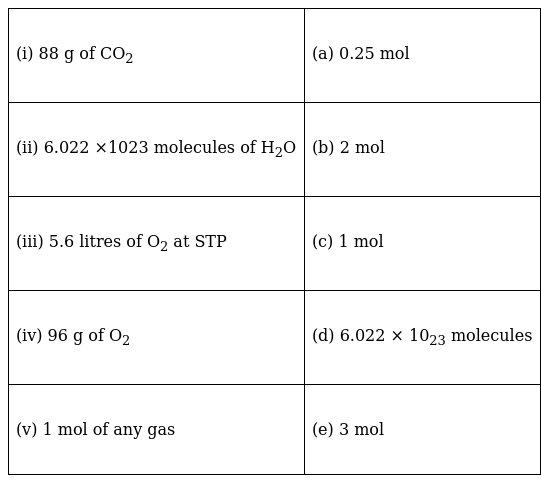
(i) 44g of CO2 constitutes 1 mol of CO2. Therefore, 88g of CO2 is 2 mol of CO2. The correct answer is (b).
(ii) 1 mol of any compound contains 6.022 x 1023 molecules of the compound. Hence 6.022 x 1023 molecules of H2O constitute 1 mol of H2O. The correct answer is (c).
(iii) At STP, 22.4L of O2 makes 1 mol of O2. Hence, 5.6L of O2 constitutes 5.6/22.4 = 0.25mol of O2. The correct answer is (a).
(iv) 1 mol of O2 contains 32g of O2. Hence 96g of O2 makes 96/32 = 3 mol of O2. The correct answer is (e).
(v) 1 mol of any gas contains 6.023 x 1023 molecules. Hence, the correct answer is (d).
1