Match the electronic configuration with electron gain enthalpy.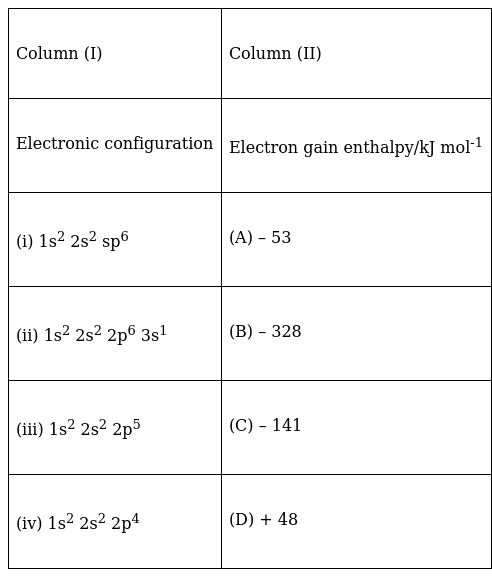
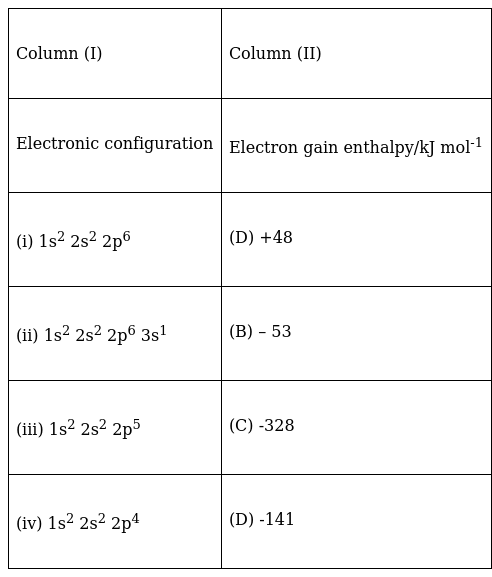
EXPLANATION
Electron gain enthalpy of elements having fully filled orbitals will be extremely positive as they do not want to lose their stability. So in the first case it is +48kJmol-1. In the second case s orbital has just one electron so this element has more tendency to donate electron but as it can also gain one electron to complete its s orbital so it has a low negative electron gain enthalpy. In the third case just one electron is required to fill the p orbital. Hence it has a very high negative electron gain enthalpy. In the fourth case as 2 more electrons are required so it has a negative electron gain enthalpy but it is less negative than the third case.
1