Match the items given in Column I and Column II.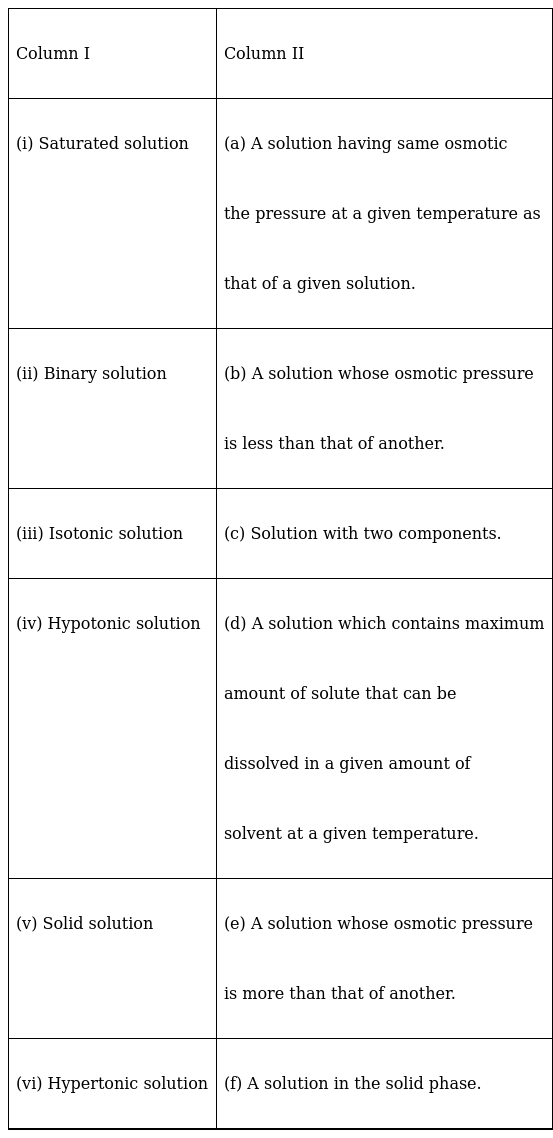
(i) Saturated solution – (d) A solution which contains the maximum amount of solute which can be dissolved in a given amount of solvent at a particular temperature is called as saturated solution
Explanation: When a maximum amount of solute has been added to a solvent at a given temperature, then this solution is said to be saturated.
For example, let’s say we keep dissolving salt in water at a given temperature. After a maximum amount has been reached, we can no longer dissolve more salt. Then this solution is said to be saturated.
When the amount exceeds this maximum amount of solute, the solution is said to supersaturate.
(ii) Binary solution- (c) Solution with two components
Explanation: Binary means two. A binary solution contains two components, each of which might be a solid, liquid or a gas.
(iii) Isotonic solution –(a)Solution having the same osmotic pressure at a given temperature as that of a given solution.
Explanation: Isotonic solutions are two solutions having the same osmotic pressure. There is no pressure difference, so the solutions can move across and mix with each other easily.
(iv) Hypotonic solution – (b) A solution whose osmotic pressure is less than that of another.
Explanation:Hypo means less. A hypotonic solution has less osmotic pressure than that of another solution. It is very dilute, i.e. it has less solute dissolved compared to another solution.
(v) Solid solution – (f) A solution in solid phase
Explanation: When two or more components form a homogenous solution in the solid state then they form a solid solution. For example, metal alloys are an amalgamation of two solid components.
(vi) Hypertonic solution – (e) A solution whose osmotic pressure is more than that of another.
Explanation:Hypertonic solutions have more solutes dissolved in them which makes them more concentrated. This gives rise to higher osmotic pressure.