Match the laws given in Column I with expressions given in Column II.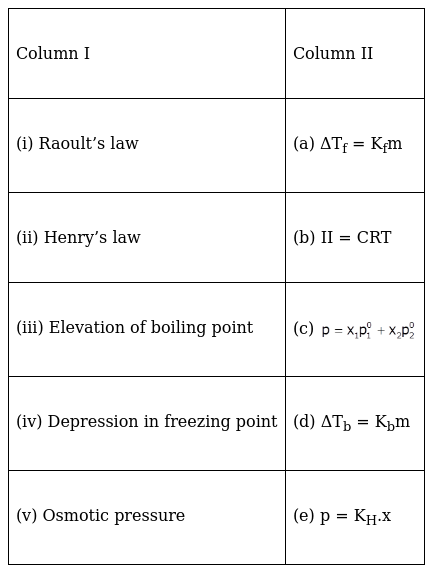
(i) Raoult’s law- (c) p=x1p1° + x2p2°
Explanation: Raoult’s law states that for a solution of volatile liquids, the partial pressure of each component of the solution is directly proportional to its mole fraction present in solution.
p=x1p1° + x2p2°
where p1° is the vapour pressure of pure component 1,
x1 is the mole fraction of component 1.
p2° is the vapour pressure of pure component 2,
x2 is the mole fraction of component 2
and p is the total pressure over the solution phase
(ii) Henry’s law – (e) p=KH.x
Explanation: Henry’s law states that at a constant temperature, the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas present above the surface of liquid or solution.
p=KH.x
where KH is Henry’s law constant,
x is the mole fraction of gas in solution and
p is the partial pressure of the gas in the vapour phase.
(iii)Elevation of the boiling point – (d) ∆Tb=Kbm
Explanation:
In the presence of non-volatile solute, the vapour pressure of the solution decreases. To reach the boiling point, vapour pressure should be increased to equal with atmospheric pressure, by increasing the temperature. This leads to an increase in the boiling point. Elevation in boiling point is measured as,
∆Tb=Kbm
Where ∆Tb is the elevation in boiling point
Kb is the boiling point elevation constant,
m is the molality of the solution
(iv)Depression in freezing point- (a) ∆Tf=Kfm
Explanation:
When the vapour pressure of solution equals vapour pressure of pure solid, then the solution will freeze. When a non- volatile solid is added to the solution, it lowers the vapour pressure which brings down the freezing point. Depression in freezing point can be calculated as,
∆Tf=Kfm
Where ∆Tf is the depression in freezing point
Kf is the freezing point depression constant
m is the molality of the solution
(v)Osmotic pressure-(b)π = CRT
Explanation: The excess pressure applied to the solution side to stop the flow of solvent molecules through the semi-permeable membrane is called osmotic pressure. Osmotic pressure can be represented as,
π = CRT
where, π is the osmotic pressure
C is the molarity of the solution
T is the temperature
R is the gas constant