Match the species in Column I with the type of hybrid orbitals in Column II.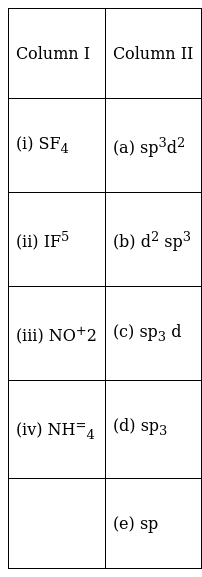
(i)![]() (c);
(c);
The hybridisation of the S is sp3d.
No. of Hybrid Orbitals = ![]() (V+M-C+A)
(V+M-C+A)
= ![]() (6+4)
(6+4)
= 5(As it has 5 hybridised orbitals).
So, it’s hybridisation is sp3d.
(ii)→ (a);
The hybridization of the I is sp3d2.
Iodine has valency = 7.
No. of Hybrid Orbitals = ![]() (7+5)
(7+5)
= 6
Thus, It’s hybridisation is sp3d2.
![]() (e);
(e);
As, there is 1 (+) charge.
No. of Hybrid Orbitals = ![]() (5-1)
(5-1)
=2.
The hybridization of the N is sp
(iv) ![]() (d);
(d);
As, there is 1 cation.
No. of hybrid orbitals = ![]() (5+4-1)
(5+4-1)
= 4.
The hybridization of the N is sp3
1