Match the species in Column I with the geometry/shape in Column II.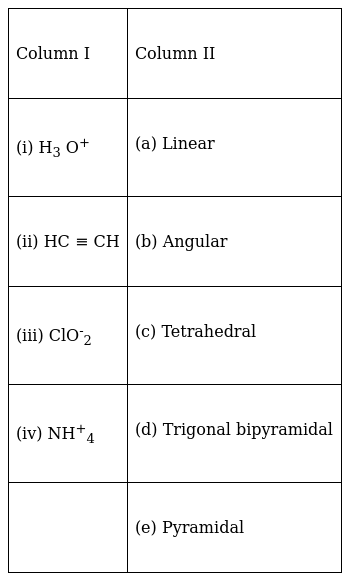
(i) ![]() (e);
(e);

The shape of H3O+ is Tetrahedral. The hybridization is sp3.
(ii) ![]() (a);
(a);

As Ethylene has double bond in its structure.
So, Ethylene is linear in shape. The hybridization is sp.
(iii) ![]() (b);
(b);

ClO-2 is angular in structure due to lone pair and bond pair repulsion.
(iv) ![]() (c);
(c);

NH+4 is tetrahedral in shape and the hybridization of N is sp3.
1