Match the shape of molecules in Column I with the type of hybridisation in Column II.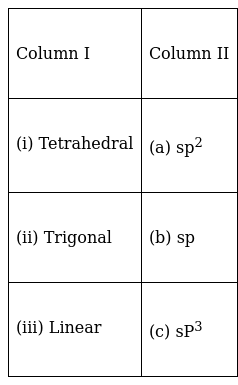
(i) ![]() (c);
(c);
the sp3 hybridized molecules have a tetrahedral shape.

(ii) ![]() (a);
(a);
the sp2 hybridized molecules have a trigonal shape.

(iii) ![]() (b);
(b);
the sp hybridized molecules have a linear shape.

1