Match structures given in Column I with the type of detergents given in Column II.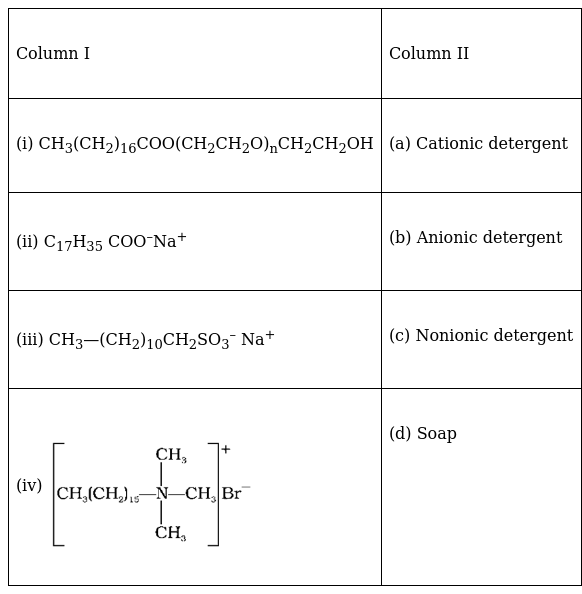
(i) CH3(CH2)16COO(CH2CH2O)nCH2CH2OH - (c) Nonionic detergent
Detergent involves surfactants (compounds which lower the surface tension between two substances – solid or liquid or both of them). As we all know, they have cleaning properties and so they are used in laundries. Their properties are very similar to that of soaps but soaps are comparatively less soluble in water.
Detergents are classified depending upon the electrical charge present on the surfactant.
Non-ionic detergents, thus from the name we get to know that the surfactant present on them is uncharged. CH3(CH2)16COO(CH2CH2O)nCH2CH2OH thus we can see the surfactant is uncharged, so it is therefore a non-ionic detergent.
(ii) C17H35 COO–Na+ - (d) Soap
As we know, soaps are cleaning agents. They are usually made by reacting alkali (eg. sodium hydroxide) with naturally occurring fat or fatty acids. Therefore we get salts of fatty acids.
Soaps have the general formula (RCO2−)nMn+ (Where R is an alkyl, M is a metal and n is the charge of the cation).
The given compound is sodium stearate - C17H35 COO–Na+ . It satisfies the general formula of soap which is (RCO2−)nMn+ . R is -C17H35 group, M is Na and n is 1. So this is an example of soap.
Sodium stearate is produced by reacting stearic acid(fatty acid) with sodium hydroxide(alkali). The reaction is as follows :-

(iii) CH3—(CH2)10CH2SO3– Na+ - (b) Anionic detergent
Detergent involves surfactants (compounds which lower the surface tension between two substances – solid or liquid or both of them). As we all know, they have cleaning properties and so they are used in laundries. Their properties are very similar to that of soaps but soaps are comparatively less soluble in water.
Detergents are classified depending upon the electrical charge present on the surfactant.
Anionic detergents have the surfactant group as sulphonates. In the compound CH3—(CH2)10CH2SO3– Na+, we can see the presence of charge, along with that it has the presence of an anionic sulphonate group. Thus, the given molecule is an anionic detergent.

(iv) - (a) Cationic detergent.
Detergent involves surfactants (compounds which lower the surface tension between two substances – solid or liquid or both of them). As we all know, they have cleaning properties and so they are used in laundries. Their properties are very similar to that of soaps but soaps are comparatively less soluble in water.
Detergents are classified depending upon the electrical charge present on the surfactant.
Cationic detergents have the cationic surfactant as a quaternary ammonium group.
In the compound 
we can see the presence of charge, along with that it has the presence of a cationic quaternary ammonium group. Thus, the given molecule is an cationic detergent.