How would you account for the following:
(i)H2S is more acidic than H2O.
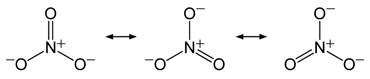
(ii) The N-O bond in NO2– is shorter than the N-O bond in NO3–
(iii) Both O2 and F2 stabilize high oxidation states but the ability of oxygen to stabilize the higher oxidation state exceeds that of fluorine.
(i) Acidic nature is determined by the ability to donate H+ ion in solution.
S lies below O in the periodic table, so S is a much larger atom than O. As O is small in size, it is firmly attached, due to which energy required to break the O-H bond becomes more and therefore it does not readily donate H+, which is not so for S atom due to its large size. As a result, S-H bond length increases and hence the bond dissociation energy of S-H is less than O-H. Therefore S-H easily loses H+ and is more acidic than H2O.
(ii) Bond order is the number of chemical bonds between a pair of atoms and indicates the stability of a bond.
If we see the structures of NO2- and NO3-, we can say that the N-O bond in NO2- has effective bond order of 2.0 whereas in NO3-, bond order is ![]() . As bond length increases with decreasing bond order, so the N-O bond in NO2– is shorter than the N-O bond in NO3–.
. As bond length increases with decreasing bond order, so the N-O bond in NO2– is shorter than the N-O bond in NO3–.
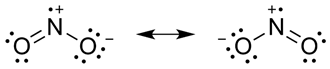
(iii) Both O2 and F2 stabilize high oxidation states but the ability of oxygen to stabilize the higher oxidation state exceeds that of fluorine. This is because, if we see both these molecules we can say that each atom of O2 bears -2 charge but each atom of F2 bears only -1 charge. As a result the attraction with a metal would be higher for O2 than F2. Due to this reason, oxygen can form multiple bonds with the metal atoms and thus stabilizes the higher oxidation state even more than fluorine.