Match the polymers given in Column I with the preferred mode of polymerisation followed by their monomers.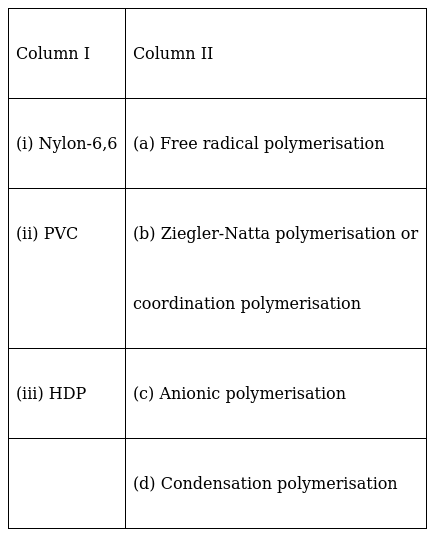
(i) Nylon-6,6 – (d) Condensation polymerisation
Nylon-6,6 is a copolymer of hexamethylenediamine and adipic acid through condensation polymerisation. The polymerisation type is named such because after the end of the reaction a simple molecule(here, H2O) is released.

(ii)PVC – (a) Free radical polymerisation
PVC is formed from vinyl chloride monomer through free radical mechanism. Vinyl polymerisation proceeds in three steps:
(1) Chain initiation- The radical initiator is broken into the intermediate free radicals by various means(eg. Sunlight or heat).
(2) Chain propagation – The free radicals combine with monomers to form bigger intermediate species.
(3) Chain terminating – The radical intermediates join with one another with the help of chemical bonds.

(iii) HDP – (b) Ziegler-Natta polymerisation or
coordination polymerization
HDP is formed when addition polymerisation of ethene takes place in a solvent in presence of catalyst like (C2H5)3Al and TiCl4 (Zeigler-Natta catalyst) at a temperature range of 333K to 343K under 6-7atm pressure.
So this process is also termed as Ziegler-Natta polymerization.
The reaction is as follows:
