Match the items of Column I and Column II.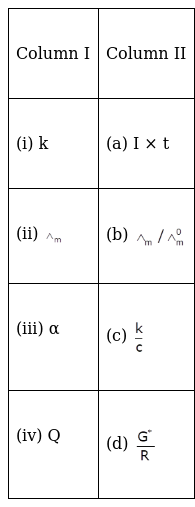
(i) K → (d) ![]()
(ii) Λm → (c) ![]()
(iii) ɑ →(b) ![]()
(iv) Q → (a) I × t
(i) We know, Resistance (R) = ρ ![]() =
= ![]()
Or, R κ = ![]() = G*
= G*
Or, κ= ![]()
Where, ρ = Resistivity or specific resistance = ![]()
L= Distance between the electrodes = length
A= Area of cross section of the electrode
G*= ![]() = Cell constant
= Cell constant
(ii) Molar conductivity, Λm is defined as the conductivity of electrolyte divided by the molar concentration of the electrolyte.
Mathematically,
Λm = ![]()
Where, κ= Conductivity of the solution
C= Concentration
(iii) Degree of dissociation, α is defined as the ratio of the molar conductivity at any concentration to the limiting molar conductivity of an electrolyte.
Mathematically,
α = ![]()
(iv) Electric charge, Q is defined as the product of the electrical current passed and the duration.
Mathematically, Q = I x T