Match the statement given in Column I with the phenomenon given in Column II.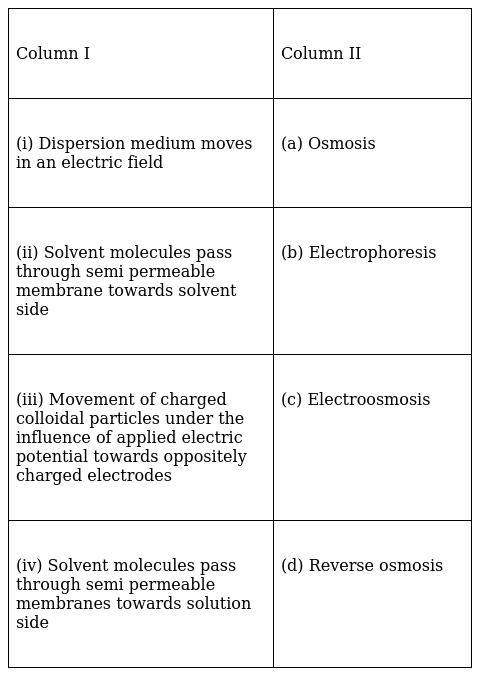
(i) → (c)
(ii) → (d)
(iii) → (b)
(iv) → (a)
EXPLANATION:
(i) Electroosmosis is the motion of liquid induced by an applied potential across a porous material, membrane, etc. The dispersion liquid moves according to the electric field. So the solvent molecules pass through semi permeable membrane towards solvent side.
(ii) Reverse osmosis is the process of forcing a solvent from a region of high solute concentration to a region of low-solute concentration through a semipermeable membrane by applying a pressure in excess of the osmotic pressure.
(iii) Electrophoresis is a separations technique that is based on the mobility of ions in an electric field. Positively charged ions migrate towards a negative electrode and negatively charged ions migrate toward a positive electrode.
So, it is basically the movement of charged colloidal particles under the influence of applied electric potential towards oppositely charged electrodes.
(iv) Osmosis is the process where solvent molecules move through a semipermeable membrane from a dilute solution into a more concentrated solution. So, the solvent molecules pass to the solution side under a semi permeable membrane.