Match the types of colloidal systems given in Column I with the name given in Column II.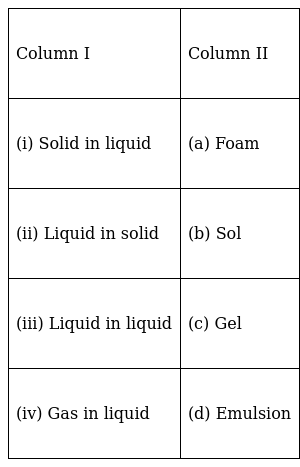
(i) → (b)
(ii) → (c)
(iii) → (d)
(iv) → (a)
Exp:
(i) A sol is a colloid made out of very small solid particles in a continuous liquid medium. Here the dispersion medium is liquid and the dispersed phase is liquid.
(ii) A gel is a solid jelly-like material that can have properties ranging from soft and weak to hard and tough. Here, in gels, dispersion of molecules of a liquid within a solid in which liquid particles are dispersed in the solid medium.
(iii) An emulsion is a special type of mixture made by combining two liquids that normally don't mix (immiscible).It is a colloid of two or more immiscible liquids where one liquid contains a dispersion of the other liquids. This process of turning a liquid mixture into an emulsion is called emulsification.
(iv) Foam is a colloidal system where the particles are gas bubbles and the medium is a liquid. Here the dispersion medium is liquid and the dispersed phase is gas. They are made by trapping air or gas bubbles inside a solid or liquid.