Match the structures of the compounds given in Column I with the name of the compounds given in Column II.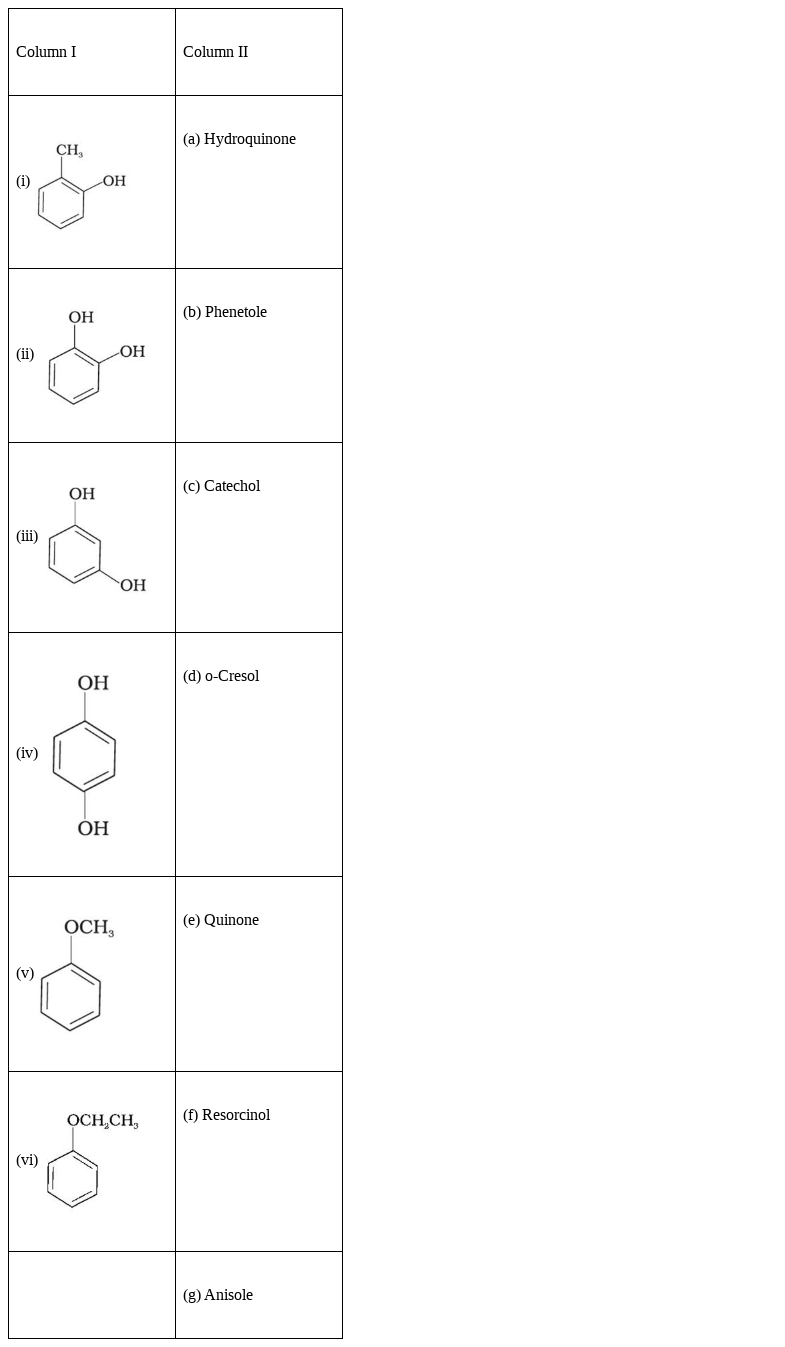
(i) ) - (d) o-Cresol
- (d) o-Cresol
So what are cresols? Cresols are nothing but methyl phenols. It means that a methyl group has substituted a Hydrogen atom in a phenol molecule.
Now, depending upon the position of the methyl group with respect to the –OH group of phenol, the compound name is given accordingly. For eg. if the methyl group is situated just adjacent to the –OH group of phenol, it is ortho cresol(o-cresol). IUPAC name is 2-methylphenol.

If the methyl group occupies the position next to the ortho, the compound is meta cresol(m-cresol). IUPAC name is 3-methylphenol.

If the methyl group is situated vertically opposite to the –OH group of phenol, the compound is called para cresol(p-cresol). IUPAC name is 4-methylphenol.

So, we can conclude that the structure of the compound given to us is o-cresol.
(ii)
 - (c) Catechol
- (c) Catechol
So what are catechols? Catechols are nothing but a part of benzene-diols. So they are formed when a hydroxyl group(-OH) substitutes a Hydrogen atom in a phenol molecule at the ortho position . Thus they are named diols due to the presence of two –OH groups in the molecule – one present phenol and the other, the substituted one at o-position. IUPAC name for catechol is 1,2-dihydroxybenzene. It is used in the production of pesticides, perfumes and pharmaceuticals.
 Catechol.
Catechol.
(iii)
 - (f) Resorcinol.
- (f) Resorcinol.
Resorcinols are somewhat similar to catechols. They also belong to the family of benzene-diols.
As we discussed earlier that catechols are ‘ortho substituted hydroxy phenols’. Similarlyresorcinols are also hydroxyl phenols, but they are meta substituted. So they are formed when a hydroxyl group(-OH) substitutes a Hydrogen atom in a phenol molecule at the metaposition . Thus they are named diols due to the presence of two –OH groups in the molecule – one present phenol and the other, the substituted one at m-position. IUPAC name for catechol is 1,3-dihydroxybenzene. It is used to treat acne and various other skin disorders.
 Resorcinol.
Resorcinol.
(iv)
 - (a)Hydroquinone
- (a)Hydroquinone
Hydroquinones are similar to that of resorcinols and catechols. As we know that resorcinols are meta substituted hydroxyl phenol and catechols are ortho substituted hydroxyl phenols, similarly hydroquinones belong to the family of benzene-diols.
They are para substituted hydroxyl phenols. So they are formed when a hydroxyl group(-OH) substitutes a Hydrogen atom in a phenol molecule at the paraposition . Thus they are named diols due to the presence of two –OH groups in the molecule – one present phenol and the other, the substituted one at p-position. IUPAC name for catechol is 1,4-dihydroxybenzene.
Its other names are benzene-1,4-diol and quinol. It is a white granular solid.
Hydroquinone is a depigmenting agent used to lighten areas of darkened skin such as freckles, age spots, chloasma, and melisma caused by pregnancy, birth control pills, hormone medicine, or injury to the skin. However, they are banned in some parts of the world as it is found to be a carcinogen or cancer-causing chemical.
 Hydroquinone.
Hydroquinone.
(v)
 - (g) Anisole
- (g) Anisole
Anisole is nothing but methoxybenzene. So this compound is an ether.
It is formed by the substitution of a Hydrogen atom from a benzene molecule by a methoxy group(-OCH3). It is a colorless liquid with a smell reminiscent of anise seed, and in fact many of its derivatives are found in natural and artificial fragrances.
 Anisole.
Anisole.
(vi)
 - (b) Phenetole
- (b) Phenetole
Similar to anisole, phenetole is also an ether. More precisely, phenetole is ethoxybenzene. ether. It is formed by the substitution of a Hydrogen atom from a benzene molecule by a ethoxy group(-OC2H5).
It is also known as ethylphenyl ether. It is volatile in nature and is explosive. It will dissolve in many non-polar compounds e.g. ethanol or ether but not in polar substances like water. Anisole is a colorless to yellowish oily liquid.
 Anisole.
Anisole.