Match the starting materials given in Column I with the products formed by these (Column II) in the reaction with HI.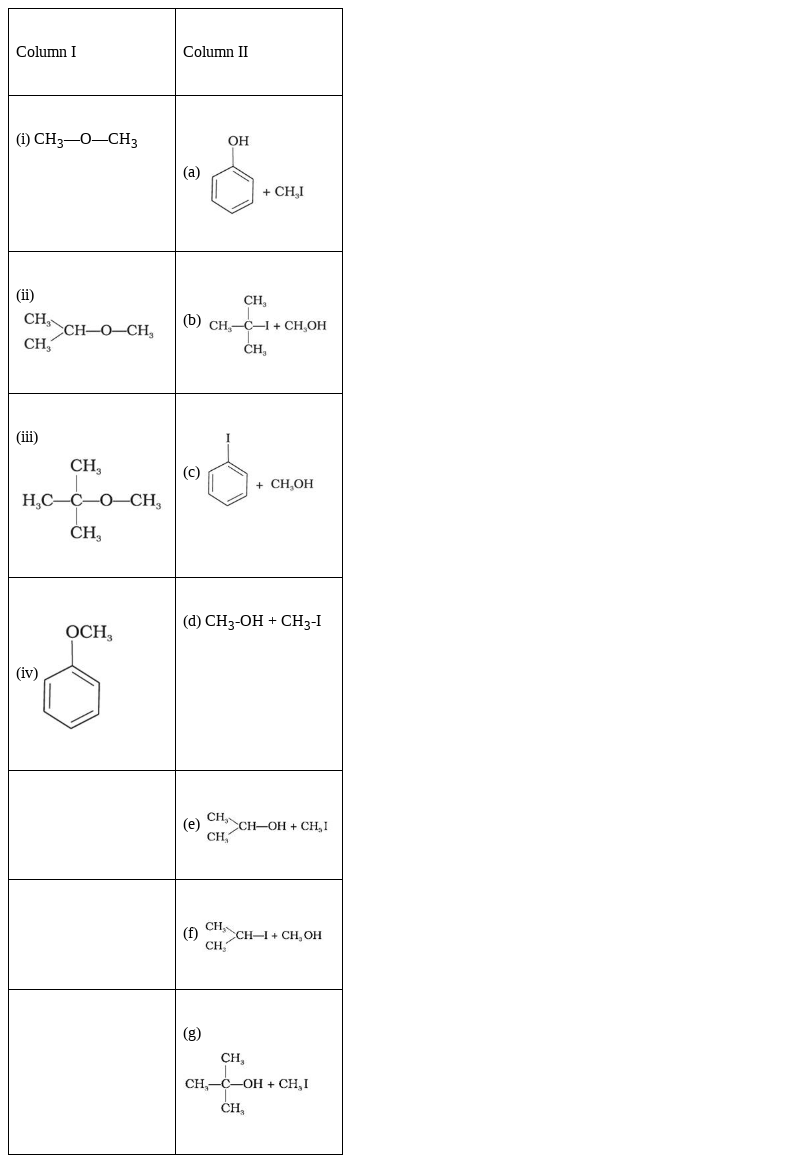
(i) CH3—O—CH3 - (d) CH3-OH + CH3-I
When methoxymethane(CH3OCH3) reacts with HI, methanol(CH3OH) and methyl iodide(CH3I) are produced.

If we want to see how it happened then here it goes. CH3OCH3 , the reactant breaks into CH3O- and CH3+ (due to more electronegative Oxygen atom, negative charge is acquired) . Similarly HI breaks into H+ and I- (as Iodine is more electronegative than Hydrogen). Now, CH3O- attacks H+ to form CH3OH and CH3+ attacks I- to form CH3I as unlike charges attract. This is the explanation to how this reaction proceeds.
(ii)
 - (e)
- (e) ![]()
When 2-methoxypropane( ) reacts with HI, 2-hydroxypropane(
) reacts with HI, 2-hydroxypropane(![]() ) and methyl iodide(CH3I) is produced.
) and methyl iodide(CH3I) is produced.

Now lets see what happens in the reaction. 2-methoxypropane( ) breaks into a stable 2° carbanion(
) breaks into a stable 2° carbanion(![]() ) and CH3+ . This is because due to the –I inductive effect of the –CH3 group the negative charge on the electronegative Oxygen atom increases. Similarly HI breaks into H+ and I- (as Iodine is more electronegative than Hydrogen). Now, the carbanion
) and CH3+ . This is because due to the –I inductive effect of the –CH3 group the negative charge on the electronegative Oxygen atom increases. Similarly HI breaks into H+ and I- (as Iodine is more electronegative than Hydrogen). Now, the carbanion ![]() attracts the H+ to form 2-hydroxypropane(
attracts the H+ to form 2-hydroxypropane(![]() ) and CH3+ attacks I- to form CH3I as unlike charges attract. This is the explanation to how this reaction proceeds.
) and CH3+ attacks I- to form CH3I as unlike charges attract. This is the explanation to how this reaction proceeds.
(iii)
 - (b)
- (b) 
When tert-butyl methyl ether( ) reacts with HI, tert-butyl iodide (
) reacts with HI, tert-butyl iodide ( )is produced along with methanol (CH3OH).
)is produced along with methanol (CH3OH).
Now, lets see what happens in this reaction.
tert-butyl methyl ether( ) breaks into a very stable tertiary(3°) carbocation, which is the tert-butyl carbocation(
) breaks into a very stable tertiary(3°) carbocation, which is the tert-butyl carbocation( ) and methoxy ion(CH3O-). Similarly HI breaks into H+ and I- (as Iodine is more electronegative than Hydrogen).
) and methoxy ion(CH3O-). Similarly HI breaks into H+ and I- (as Iodine is more electronegative than Hydrogen).
As we know –CH3 groups show –I inductive effect, due to the presence of 3 such methyl groups the delta positive charge on the carbocation starts diminishing due to which the carbocation becomes highly stable. Thus it would now prefer the attack of the I- ion and it would result in the formation of tert-butyl iodide ( ). Along with that the methoxy ion(CH3O-) reacts with H+ to form methanol (CH3OH) as unlike charges attract. This is the explanation to how this reaction proceeds.
). Along with that the methoxy ion(CH3O-) reacts with H+ to form methanol (CH3OH) as unlike charges attract. This is the explanation to how this reaction proceeds.
Note:
tert stands for tertiary.
(iv)
 - (a)
- (a) 
When anisole ( ) reacts with HI, phenol (
) reacts with HI, phenol ( ) and methyl iodide (CH3I) are produced.
) and methyl iodide (CH3I) are produced.
Lets see how the reaction goes. Anisole breaks down into phenoxide ion (C6H5O-). As we know, phenoxide ion is resonance stabilized , the formation of the phenoxide ion is highly preferred. So we get to know that any substance always want to acquire a state where it gains maximum stability.

Along with that CH3+ carbocation is also formed. Similarly HI breaks into H+ and I- (as Iodine is more electronegative than Hydrogen).
Now, the phenoxide ion( ) reacts with H+ to form a well known compound called phenol (
) reacts with H+ to form a well known compound called phenol ( ). Also CH3+ carbocation andI- interact amongst themselves to form methyl iodide(CH3I) as unlike charges attract. This is the explanation to how this reaction proceeds.
). Also CH3+ carbocation andI- interact amongst themselves to form methyl iodide(CH3I) as unlike charges attract. This is the explanation to how this reaction proceeds.