Match the following rules with their statements :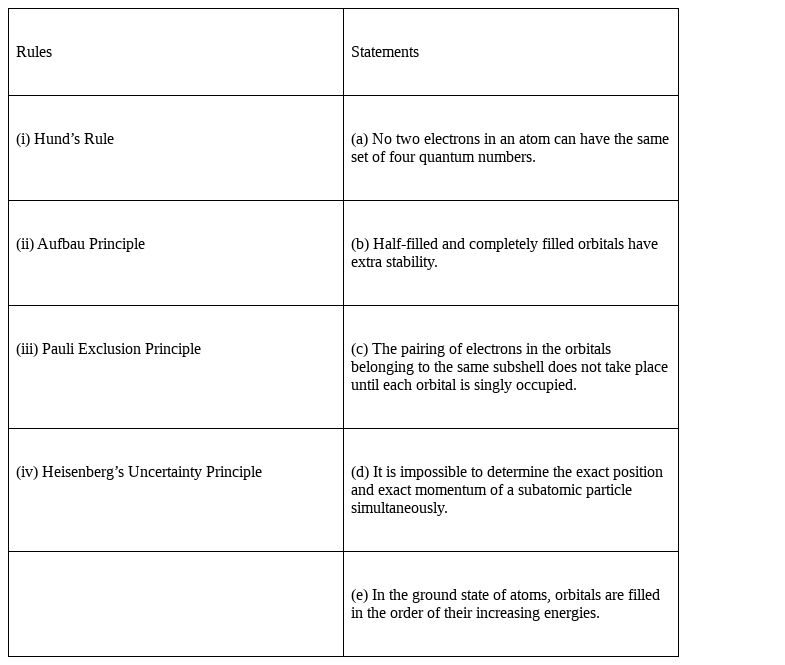
Matching the following rules with their statements:
Hund’s Rule – (c) Pairing of electrons in the orbitals belonging to the same subshell does not take place until each orbital is singly occupied.
And also
(b) Half-filled and completely filled orbitals have extra stability.
(ii) Aufbau Principle –(e) In the ground state of atoms, orbitals are filled in the order of their increasing energies.
(iii) Pauli Exclusion Principle -(a) No two electrons in an atom can have the same set of four quantum numbers.
(iv) Heisenberg’s Uncertainty Principle -(d) It is impossible to determine the exact position and exact momentum of a subatomic particle simultaneously.
1