The solubility product of Al(OH) 3 is 2.7 × 10-11. Calculate its solubility in gL-1 and also find out the pH of this solution. (Atomic mass of Al = 27 u).
Given, Ksp =2.7×10-11
The equation of disassociation of Al(OH)3![]() will be-
will be-
![]()
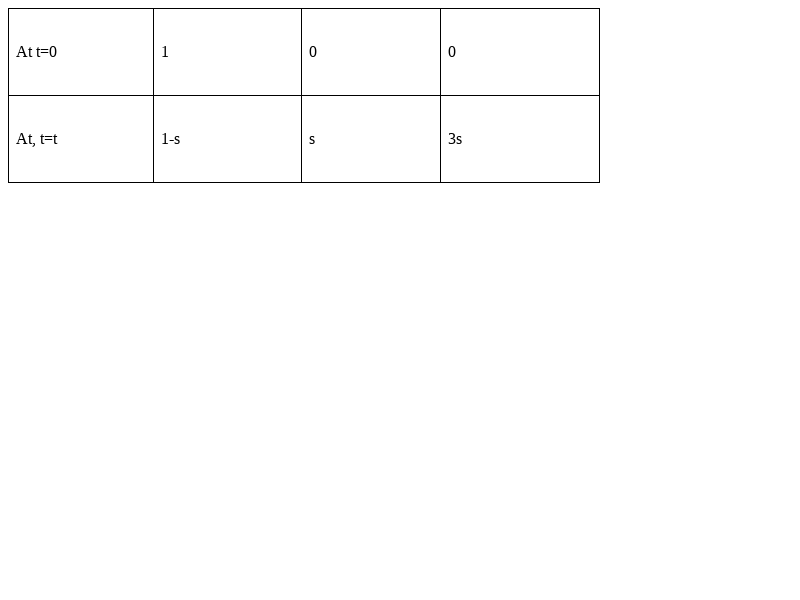
We know that,
Ksp= [Al3+] [OH-] 3-
=(s) × (3s) 3
=27s4
![]()
![]()
s4= 10-12
s= (10-12)1/4 =10-3 mol/L
Now, molar mass of Al(OH)3 =78
![]() Solubility= molar mass ×s
Solubility= molar mass ×s
= 78 ×10-3
= 7.8 × 10-2 g/L
NOW, we know that-
pH = 14 - pOH
[OH]= 3s = 3 × 10-3
pOH= 3-log3
pH = 14 – 3 + log3
= 11.4771
Hence the pH of the solution will be 11.4771 and solubility in g/L will be 7.8×10-2 g/L.
1