Calculate the volume of water required to dissolve 0.1 g lead (II) chloride to get a saturated solution. (Ksp of PbCl2 = 3.2 × 10-8, atomic mass of Pb = 207 u).
Given, Ksp of PbCl2 =3.2 ×10-8
The equation of disassociation of PbCl2 will be-
![]()
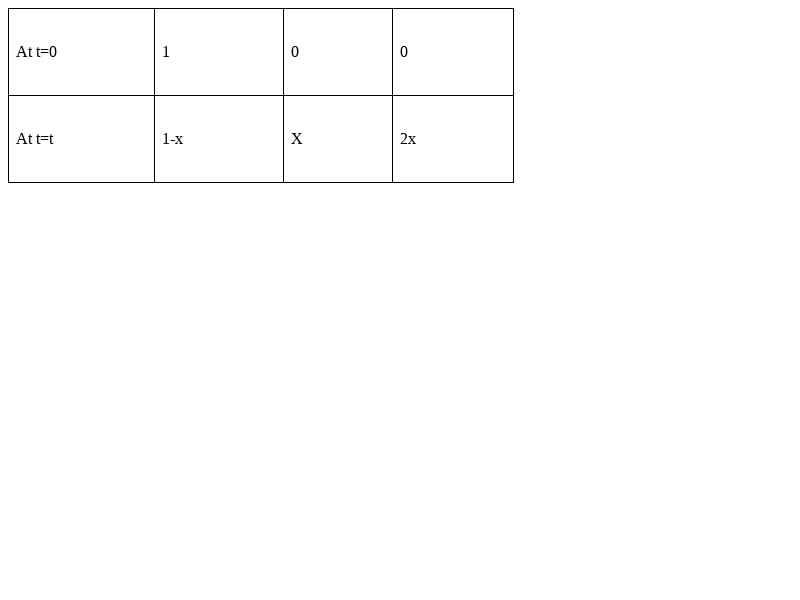
Ksp = [Pb2+] [Cl-] 2
= (x) × (2x) 2 = 4x3
4x3 =3.2 × 10-8
x=2 ×10-3 mol/L
Solubility = molar mass (PbCl2) × 2 × 10-3
=556 × 10-3 =0.556 g/L![]()
![]() The required volume to get a saturated solution of PbCl2 is 0.1798 L
The required volume to get a saturated solution of PbCl2 is 0.1798 L
1