A parallel beam of light of wavelength 100 nm passes through a sample of atomic hydrogen gas in ground state.
(a) Assume that when a photon supplies some of its energy to a hydrogen atom, the rest of the energy appears as another photon moving in the same direction as the incident photon. Neglecting the light emitted by the excited hydrogen atoms in the directions of the incident beam. What wavelengths may be observed in the transmitted beam?
(b) A radiation detector is placed near the gas to detect radiation coming perpendicular to the incident beam. Find the wavelengths of radiation that may be detected by the detector.
Given: wavelength=100nm
Energy associated with this wavelength
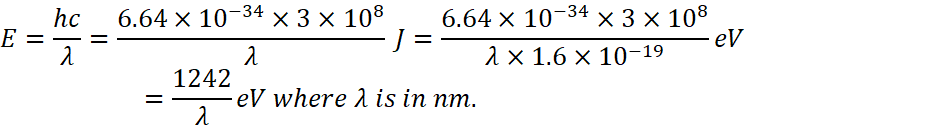
![]() (substituting the vaue of c and plank’s constant)
(substituting the vaue of c and plank’s constant)
![]()
(a)Suppose En be the energy in the nth orbit. Let consider all possible change in transitions state.
Energy absorbed in transition state in n=1 to n=2
![]()
Energy left![]()
Energy left![]() as we know that
as we know that
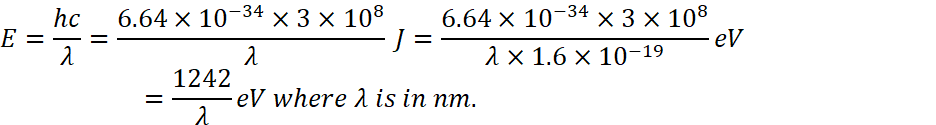
![]()
![]()
Energy absorbed in transition state in n=1 to n=3
![]()
Energy left![]()
Energy left![]()
![]()
![]()
Energy absorbed in transition state in n=3 to n=4
![]()
Energy left![]()
Energy left![]()
![]()
![]()
(b)According to the question if hydrogen atom is radiated perpendicularly then only absorbed energy to change the transition state is taken into consideration.
For n=1 to n=2
![]()
And 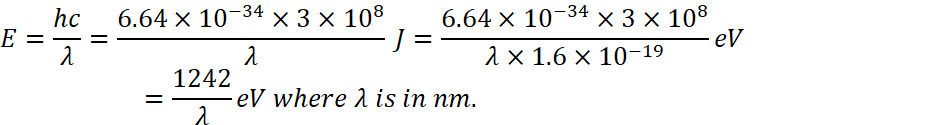
![]()
![]()
![]()
For n=1 to n=3
![]()
![]()
![]()
![]()
For n=3 to n=4
![]()
![]()
![]()
![]()
Hence three wavelength obtained are 103nm,121nm,1911nm.