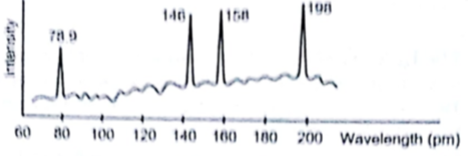
Continuous X-rays are made to strike a tissue paper soaked with polluted water. The incoming X-rays excite the atoms of the sample by knocking out the electrons from the inner shells. Characteristic X-rays are subsequently emitted. The emitted X-rays are analysed and the intensity is plotted against the wavelength figure. Assuming that only Kα intensities are detected, list the elements present in the sample from the plot. Use Moseley’s equation.
Given:
v = (25 × 1014 HZ) (Z – 1)2.
Using Moseley’s equation
v = (25 × 1014 HZ) (Z – 1)2![]()
I case
λ = 78.9× 10-12 m
Therefore,![]()
![]()
![]()
![]()
Element with z = 40 is Zr - zirconium
II case
![]()
![]()
![]()
![]()
![]()
Element with z =30 is Zn-zinc
III case
![]()
Therefore,
![]()
![]()
![]()
Element with z = 29 is Cu-copper
VI case
![]()
therefore,
![]()
![]()
Z = 25.6162 = 26(approx.)
Element with Z = 26 is Fe-Iron
The elements Present in the polluted water are Zirconium, Zinc, Copper and Iron.