A sample of 1.0 mol of a monoatomic ideal gas is taken through a cyclic process of expansion and compression as shown in Fig. 6.1. What will be the value of ΔH for the cycle as a whole?
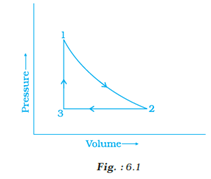
Enthalpy or H is a state function, which means it does not depend on the path on which a process is carried out. Instead, it depends on the initial and final state of a process and changes accordingly.
In the following cyclic ( 1 → 2 →3 →1 ) process the initial and final point is the same (i.e. 1). Hence the enthalpy change or ∆H= 0, in other words, there will be no change in enthalpy.
1