Match the following :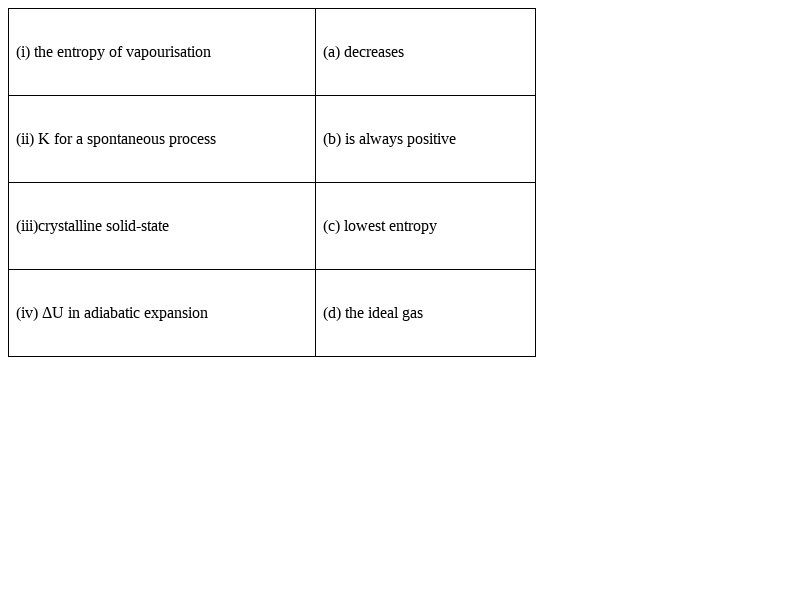
(i)Entropy of vapourisation – (b)is always positive
Explanation: When the liquid vaporizes, the degree of the disorder increases, therefore, entropy is always positive.
(ii) K for the spontaneous process- (d) the ideal gas
Explanation: K is equilibrium constant and is always positive.∆rH- is large and positive for strong endothermic reaction and the value of K is smaller than 1.
∆rH- is large and negative for exothermic reaction and the value of K is larger than 1. ∆rG- is also negative and large.
To get a large value of K we need a strongly exothermic reaction, hence we go for completion of the reaction.
∆rG- depend on ∆rS- and if entropy changes then the value of K also get affected, which depend on whether ∆rS- is positive or negative.
(iii) Crystalline solid-state : (c) lowest entropy
Explanation: Crystalline solid state is the most ordered form so it has the lowest entropy and gaseous state is disordered state so highest entropy.
(iv) ΔU in adiabatic expansion – (a) decreases
Explanation, as there is no exchange of heat between system and surrounding, internal energy will decrease after applying some amount of pressure.