Match the complex ions given in Column I with the hybridisation and numberof unpaired electrons given in Column II and assign the correct code: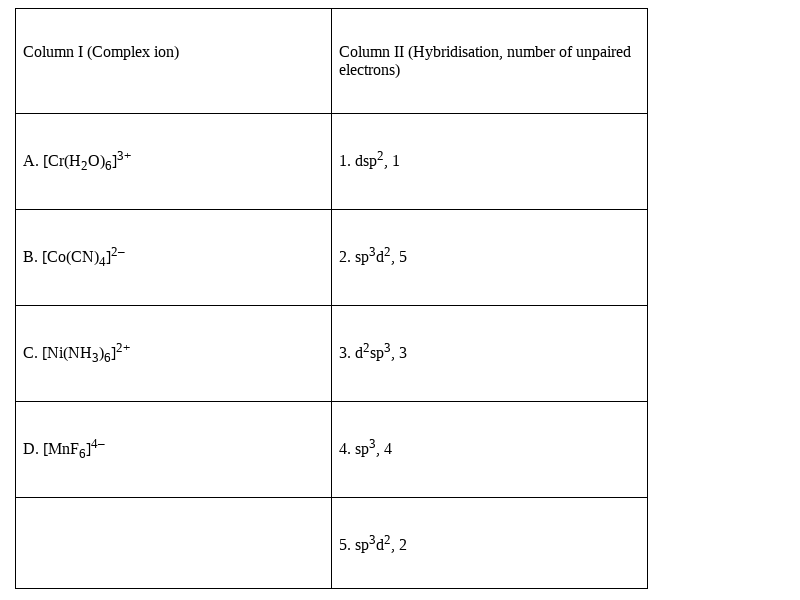
Code:
• In the case of A. [Cr(H2O)6]3 +: The central metal atom is Cr and oxidation state x=+3 as water molecules are neutral.
Therefore , having a d3 configuration (1s2 2s2 2p6 3s2 3p6 3d3).
Cr has +3 oxidation state with d3 configuration and left with 2 empty d orbitals and 1 empty 4s orbital which in interaction with 3,4p orbitals and attain the hybridisation d2sp3 and the 6 H2O electrons will occupy these 6 hybridised orbitals. And the hybridisation scheme will be :
The orbital energy diagram of [Cr(H2O)6]3+is :

Hence, there will be 3 unpaired electrons.
• In case of B. [Co(CN)4]2– : The central metal atom is Co and oxidation state x= -4 ![]() (-1) – 2=+2,
(-1) – 2=+2,
Therefore, having a d7configuration (1s2 2s2 2p6 3s2 3p6 3d7).Hence, the Co2+ is left with 1 3d orbital,1 4s orbital which in interaction with 2, 4p orbitals and attains hybridisation of dsp2and the 4CN- electrons will occupy these 4 hybridised orbitals and the scheme will be:

The orbital energy diagram will be:
 hence, there will be 1 unpaired electron.
hence, there will be 1 unpaired electron.
• In the case ofC. [Ni(NH3)6]2+: The central metal atom is Ni and oxidation state x=+2,
Therefore, having a d8 configuration (1s2 2s2 2p6 3s2 3p6 3d8).Hence, the Ni2+ is left with 2, 3d orbital,1 4s orbital which in interaction with 2, 4p orbitals and attain hybridisation of sp3d2and the 6, NH3 electrons will occupy these 6 hybridised orbitals and the scheme will be:

The orbital energy diagram will be:

hence, there will be 2 unpaired electrons.
• In the case of D. [MnF6]4–: The central metal atom is Mnand oxidation state x=-6![]() (-1) – 4 =+2
(-1) – 4 =+2
Therefore, having a d5configuration (1s2 2s2 2p6 3s2 3p6 3d5).Hence, the Mn2+ is left with 1 4s, 2 of 4d (outer orbital complex) which in interaction with 3, 4p orbitals and attain hybridisation of sp3d2and the 6, F-electrons will occupy these 6 hybridised orbitalsand the scheme will be:

hence, there will be 5 unpaired electrons as it a high spin complex.
The correct answer will be the option (i)A (3) B (1) C (5) D (2)
Hence, matching the two columns the right combination will be:
A. [Cr(H2O)6]3+ - 3. d2sp3, 3
B. [Co(CN)4]2–-1. dsp2, 1.
C. [Ni(NH3)6]2+-5. sp3d2, 2
D. [MnF6]4– -2. sp3d2, 5