Arrange the following alkyl halides in decreasing order of the rate of β– elimination reaction with alcoholic KOH.

![]()
![]()
Rate of β– elimination reaction with alcoholic KOH of the given alkyl halides depends on the stability of carbocation formed during the reaction. If the carbocation formed is stable that reaction will be faster.
Addition of alcoholic KOH results in removal of Br and H from a β carbon and a carbocation will form. β carbon is the adjacent carbon to the carbon attached with Br. Following carbocations will form-
As we know that order of stability of carbocation is 3°>2°>1°.
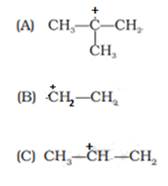
The stability of formed carbocation is A>C>B and thus rate of the reaction will also be in same order i.e. (iv) A > C > B.
1