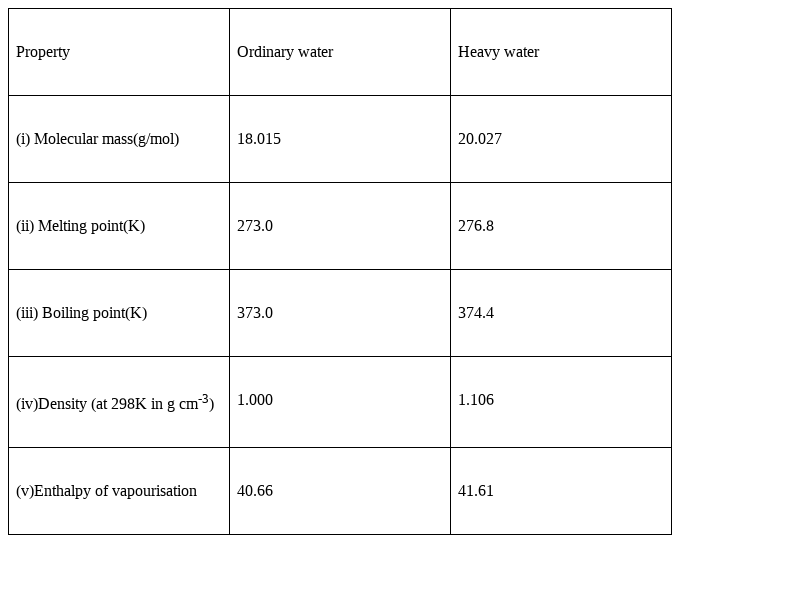
How is heavy water prepared? Compare its physical properties with those of ordinary water.
Heavy water, which is deuterium oxide (D2O) is mainly prepared by electrolysis of ordinary water.
This method involves multistage electrolysis of ordinary water containing alkali like NaOH. The cylindrical vessels made of steel which act as cathode and cylindrical sheets of nickel acts as anode having a number of holes punched in it. The electrolysis carried out in different stages as we mentioned earlier. Large number of electrolytic cell are also used for this process.
1