Melting point, enthalpy of vapourisation and viscosity data of H2O and D2O is given below :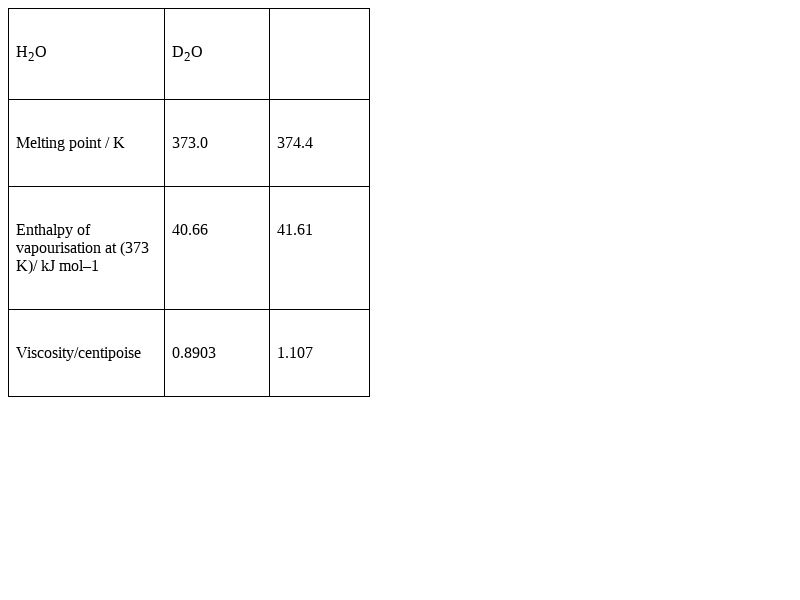
On the basis of this data explain in which of these liquids intermolecular forces are stronger?
We know, melting point, enthalpy of vapourisation, viscosity all these physical properties depend on the intermolecular forces of attraction. From the data supplied above, we can see that all the values corresponding to the properties are higher for D2O than that of H2O. So, we can say that the intermolecular forces in D2O are stronger.
1