If c is r.m.s. speed of molecules in a gas and v is the speed of sound waves in the gas, show that c/v is constant and independent of temperature for all diatomic gases.
From kinetic theory of gases, the rms speed of molecules of a gas is given by,
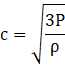
where P is the pressure of the gas and ρ is the density. From ideal gas law
![]()
Where R is the universal gas constant, T is temperature and M is the molecular mass of the gas.
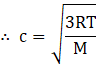
The speed of sound on the other hand is given by
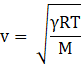
where γ is the ratio of specific heat at constant pressure to specific heat at constant volume for air, R is the universal gas constant, T is temperature and M is the molecular mass of the gas.
Therefore
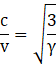
For a diatomic gas γ = 1.4, and hence
![]()
And is independent of temperature.
1