(a) Illustrate the following reactions giving suitable examples in each case:
(i) Ammonolysis
(ii) Coupling reaction
(iii) Acetylation of amine
(b) Describe Hinsberg method for the identification of primary, secondary and tertiary amines. Also write the chemical equations of the reactions involved.
OR
(a) Write the structure of main products when benzene diazonium chloride (C6H5![]() Cl-) reacts with the following reagents:
Cl-) reacts with the following reagents:
i. HBF4 /Δ
ii. Cu/HBr
(b) Write the structure of A, B and C in the following reactions:

(a) (i) Ammonolysis
On treating alkyl or benzyl halide with ethanolic solution of ammonia, amines are obtained. This is a nucleophilic substitution reaction in which ammonia acts as the nucleophile. In this process there is a cleavage of C-X bond by ammonia which is named as ammonolysis.
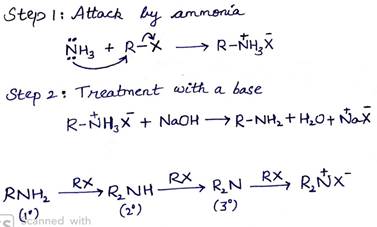
(ii) Coupling reaction
This is an electrophilic substitution reaction. When phenol reacts with benzene diazonium chloride, para position of phenol gets coupled with benzene diazonium ion to form an azo compound, p-azoxy azobenzene.
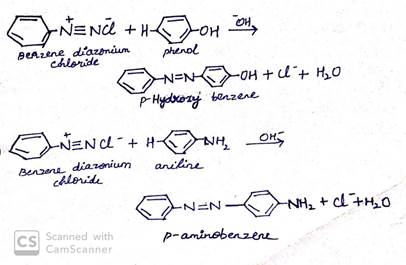
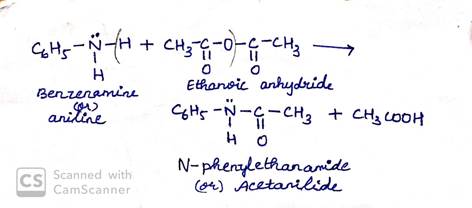
(iii) Acetylation of amine
Acetylation can be carried by treating acetic anhydride on amine.
(b) Hinsberg method for differentiating amines-
![]()
![]()
![]()

Benzenesulphonyl chloride is known as the Hinsberg reagent.
Primary amines on reacting with the reagent forms N-ethylbenzenesulphonamide. (equation (1)). This compound has acidic proton so it is soluble in alkali.
Secondary amines on reacting with the Hinsberg reagent forms N,N-diethylbenzenesulphnamide. This compound doesn’t have any acidic proton so it is not soluble in alkali. [equation (2)].

Tertiary amines do not react with benzenesulphonyl chloride.
These difference in reactivity of amines towards the Hinsberg’s reagent can help differentiate between them.
OR
(a)
(i)
![]()
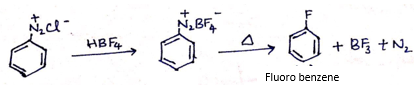
(ii)
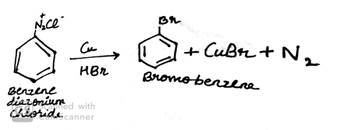
(b) Write the structure of A, B and C in the following reactions:
(i) ![]()
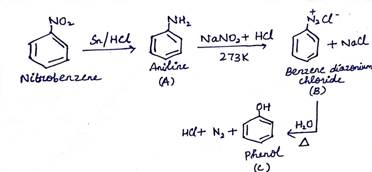
(ii) ![]()
