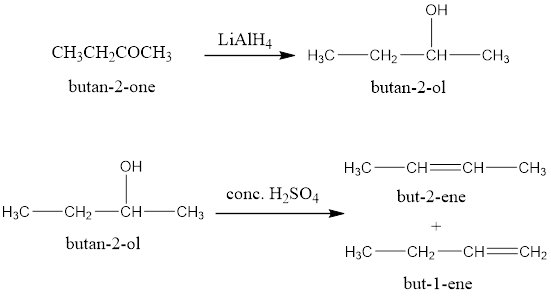
An organic compound X having molecular formula C4H8O gives orange-red precipitate with 2,4-DNP reagent. It does not reduce Tollens’ reagent but gives yellow precipitate of Iodoform on heating with NaOI. Compound X on reduction with LiAlH4 gives compound ‘Y’ which undergoes dehydration reaction on heating with conc. H2SO4 to form But-2-ene Identify the compounds X and Y.
Given molecular formula fits in the general category of aldehydes or ketones and alcohols with formula CnH2nO for n=2. The compound 2,4-DNP has the following structure:
The amine group of hydrazine has a lone pair that attacks as the nucleophile and reacts with aldehydes and ketones to give a intermolecular condensation reaction as follows:
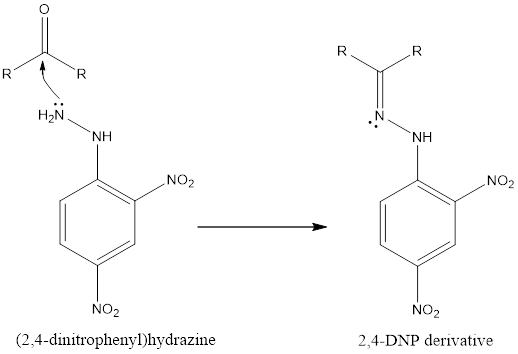
When the given compound is aliphatic the derivative has yellow colour and it gives orange-red colour for aromatic derivatives this says that it is a ketone. Also the Tollens test verifies this. Thus the compound is butanone.
When it is reduced with LiAlH4 it gives a secondary alcohol which on treatment with concentrated H2SO4 gives but-2-ene.
Rxn: