How do you convert:
(i) Chlorobenzene to toluene
(ii) Bu-1-ene to but-2-ene
(iii) Ethanol to ethyl iodide
OR
What happens when:
(i) n-butyl chloride is treated with alcoholic KOH
(ii) 2-chloropropane is treated with sodium in presence of dry ether
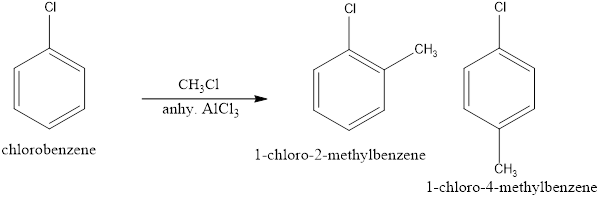
(iii) Chlorobenzene is treated with ![]() in the presence of anhydrous
in the presence of anhydrous ![]() .
.
Write the chemical equations involves in the above reactions.
Option (i) Wurtz Fittig reaction can be used to convert Chlorobenzene to toluene by making it react to sodium in presence of methyl chloride will give you toluene. 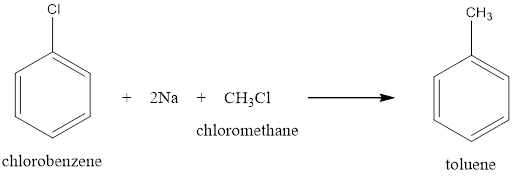
This reaction also gives intramolecular products as ethane is found as a by-product.
Option (ii)
But-1-ene can be reduced by addition of HBr which will undergo Markovnikov’s addition forming 2-bromobutane which on treatment with alcoholic KOH undergoes Saytzeff’s elimination to give but-2-ene.
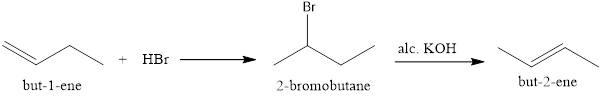
Option (iii)
Alcohols when treated with halogen halides give their corresponding halogen derivative. Thus treatment of ethanol with hydrogen iodide will give ethyl iodide.

OR
Option (i)
When n-butylchloride is treated with alcoholic KOH it undergoes β-elimination to give but-1-ene.
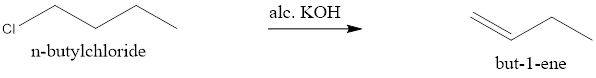
NOTE: The hydride shift can be considered as an alternative due to higher stability of 2° carbocation, but the energy required to do the 1,2-hydride shift is not sufficient so the 2° carbocation is not considered.
Option (ii)
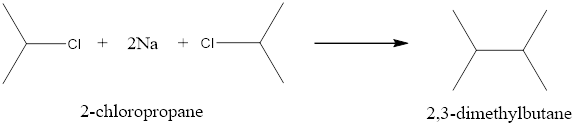
When 2-chloropropane is treated with Na in dry ether it undergoes Wurtz reaction to give 2,3-dimethyl butane as a major product.
Option (iii)
When Chlorobenzene is treated with methyl chloride in presence of anhydrous ALCl3 it undergoes Friedel Crafts alkylation which facilitates electrophilic substitution of CH3 on the benzene ring to give o-chlorotoulene and p-chlorotoulene as a product.