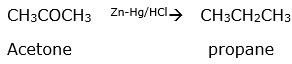Give reasons:
(i) The ![]() -hydrogen atoms of aldehydes and ketones are acidic in nature.
-hydrogen atoms of aldehydes and ketones are acidic in nature.
(ii) Propanone is less reactive than ethanol toward addition of HCN.
(iii) Benzoic acid does not give Friedal-Crafts reaction.
OR
How can you convert?
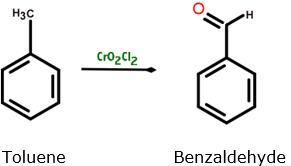
(i) Toluene to Benzaldehyde
(ii) Ethanoic acid to 2-chloroethanoic acid
(iii) Acetone to Propane
(i) The ![]() -hydrogen atoms of aldehydes and ketones are acidic in nature because the anion formed after the removal of
-hydrogen atoms of aldehydes and ketones are acidic in nature because the anion formed after the removal of ![]() -hydrogen is resonance stabilized by carbonyl group.
-hydrogen is resonance stabilized by carbonyl group.
(ii) Propanone is less reactive than ethanal toward addition of HCN because in propanone there are two methyl groups present as compared to ethanal in which only one methyl group is present. The 2 methyl group decreases the electrophilicity of carbonyl carbon due to their electron releasing nature and also the two methyl group hinders the approach of nucleophile.
(iii) Benzoic acid does not give Friedal-Crafts reaction as carboxyl group deactivates the ring and prevent the substitution at ring.
OR
(i) Etard’s reaction
(ii) Hell- Volhard- Zelinsky reaction
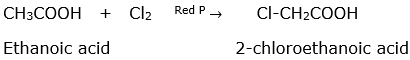
(iii) Clemmensen reduction