Write the structure of Monomers used to obtain the following polymers:
(a) Buna-S
(b) Glyptal
(c) Nylon-6
OR
(a) Arrange the following polymers in increasing order of their intermolecular forces: Polyvinylchloride, Neoprene, Terylene.
(b) Write one example each of
(i) Natural Polymer
(ii) Thermosetting polymer
(c) What is the significance of numbers 6,6 in Nylon-6,6?
(a) The monomers of Buna- S are styrene and 1,3- Butadiene.
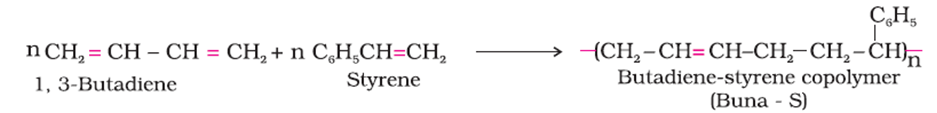
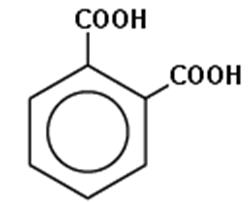
(b) The monomers of Glyptal are Ethylene glycol and Phthalic acid.
![]()
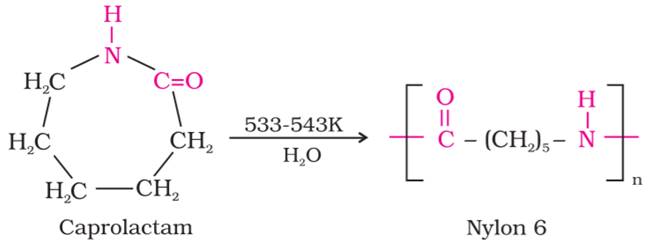
(c) The monomer of Nylon-6 is caprolactum.
OR
(a) Polyvinyl chloride is thermoplastic polymer, Neoprene is an elastomer and Terylene is a fibre. The intermolecular forces are strongest in fibres then in plastics and weakest in elastomers. Thus, polymers in increasing order of intermolecular forces are arranged as follows-
Neoprene < Polyvinyl Chloride < Terylene
(b)
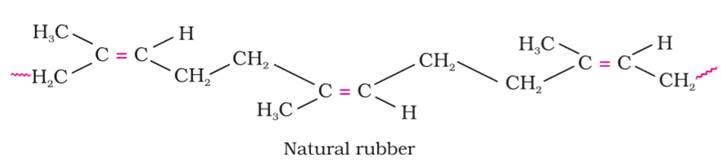
(i) Example of Natural Polymer is Rubber. Natural rubber may be considered as a linear polymer of isoprene (2-methyl-1, 3-butadiene) and is also called as cis - 1, 4 polyisoprene.
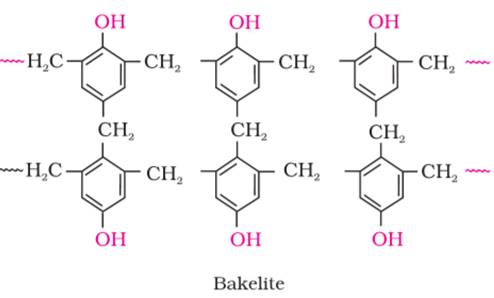
(ii) Example of Thermosetting polymer is Bakelite.
(c) Nylon 6,6 is a condensation polymer of adipic acid and hexamethylene diamine and both of these contains 6 carbon atoms. Thus, its name is Nylon 6,6.