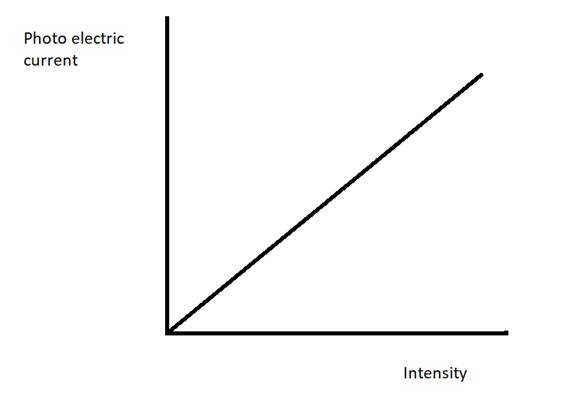
Plot a graph showing the variation of photoelectric current with intensity of light. The work function for the following metals is given: Na: 2.75 eV and Mo: 4.175 eV.
Which of these will not give photoelectron emission from a radiation of wavelength 3300 Å from a laser beam? What happens if the source of laser beam is brought closer?
OR
Define the term “cut off frequency” in photoelectric emission. The threshold frequency of a metal is f. When the light of frequency 2f is incident on the metal plate, the maximum velocity of photo-electrons is v1. When the frequency of the incident radiation is increased to 5f, the maximum velocity of photo-electrons is v2. Find the ratio v1: v2.
Given:
Wavelength of laser light, λ=3300A0
Explanation:
A graph showing the variation of photoelectric current with intensity of light is given as follows.
The energy of incident photon,
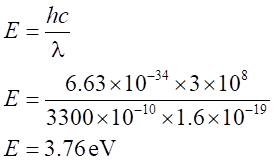
The work function of Na is less than the energy of incident photon, so photo electric emission take place for Na.
For Mo the work function is more than the incident energy. So photo electric emission will not take place for Mo.
When the source of laser is brought closer the kinetic energy of the photon remains the same but photo electric current changes.
OR
Cut off frequency is the minimum frequency of the incident radiation required to cause photoelectric current. It is also called threshold frequency.
According to Einstein’s equation of photoelectric effect we have,
![]()
For frequency = 2f,
![]() … (1)
… (1)
For frequency = 5f,
![]() … (2)
… (2)
Taking ration of equation (1) and (2) we get,
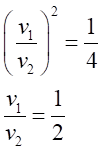
The ratio of v1: v2=1:2