Name any two elements of group one and write their electronic configurations. What similarity do you observe in their electronic configurations? Write the formula of oxide of any of the aforesaid element.
Two elements of group 1:
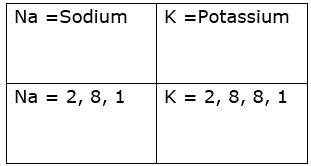
Similarity between Na and K is that both have one electron in their outermost shell. Thus both have one valence electron and valency one.
Oxide of Sodium → Na2O
Oxide of Potassium → K2O
10