When 3-methylbutan-2-ol is treated with HBr, the following reaction takes place:
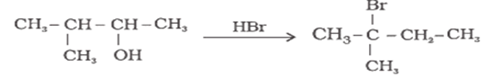
Give a mechanism for this reaction.
(Hint: The secondary carbocation formed in step II rearranges to a more stable tertiary carbocation by a hydride ion shift from 3rd carbon atom.)
The first step in the mechanism of the given reaction is protonation of the alcohol followed by loss of water to give a 20 carbocation.

The next step is a rearrangement of the 20 carbocations formed in the above step is less stable it rearranges by a 1,2-hydride shift to form more stable 3° carbocations.

The last step of the reaction is the nucleophilic attack of Br- ion on the 3° carbocations giving the final product.

33