For the following bond cleavages, use curved-arrows to show the electron flow and classify each as homolysis or heterolysis. Identify reactive intermediate produced as free radical, carbocation and carbanion.
(a) ![]()
(b) ![]()
(c) 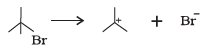
(d) 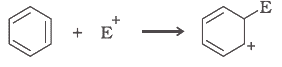
(a) The bond cleaves using curved arrows to show the electron flow of the given reaction can be represented as
![]()
It is an example of homolytic cleavage as one of the shared pair in a covalent bond goes with the bonded atom. The reaction intermediate formed is free radical.
(b) The bond cleaves using curved arrows to show the electron flow of the given reaction can be represented as

It is an example of heterolytic cleavage as the bond breaks in such a manner that the shared pair of electrons remains with the carbon of propanone. The reaction intermediate is carbanion.
(c) The bond cleaves using curved arrows to show the electron flow of the given reaction can be represented as

It is an example of heterolytic cleavage as the bond breaks in such a manner that the shared pair of electrons remains with the bromine ion. The reaction intermediate is carbocation.
(d) The bond cleaves using curved arrows to show the electron flow of the given reaction can be represented as

It is an example of heterolytic cleavage as the bond breaks in such a manner that the shared pair of electrons remains with one of the fragments. The reaction intermediate is carbcation.