H3PO3 can be represented by structures 1 and 2 shown below. Can these two structures be taken as the canonical forms of the resonance hybrid representing H3PO3? If not, give reasons for the same
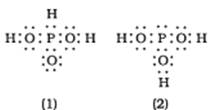

No, the structures 1 and 2 cannot be taken as canonical forms because the position of atoms has been changed.
While making resonating structures, following conditions must be followed:
⇒We cannot change the position of any atom. Atoms must be in their own position.
⇒We can only shift the bonds from one atom to another.
⇒We can only change the position of electrons from one atom to another.
12