A 10% solution (by mass) of sucrose in water has a freezing point of 269.15 K. Calculate the freezing point of 10% glucose in water if freezing point of pure water is 273.15 K.
Given : (Molar mass of sucrose = 342 g mol–1)
(Molar mass of glucose = 180 g mol–1)
Given : (Molar mass of sucrose = 342 g mol–1)
(Molar mass of glucose = 180 g mol–1)
Freezing point of sucrose solution = 269.15K = T1
Freezing point of glucose solution = 273.15 = T2
Concentrations of both solutions = 10% by mass
We know that ∆Tf = Kf ×m, where
∆Tf = depression in freezing point = T2 – T1
Kf = cryoscopic constant
We know that
If w2 is the grams of the solute having molar mass as M2, present in w1 gram of solvent, produces the depression in freezing point ΔTf of the solvent then molality of the solute m is given by the equation-
m = ![]()
assume 100g of solution containing 90% solvent and 10% solute
mass of solute (sucrose) = w2 = 10% of 100 = 10g
molar mass of sucrose = M2 = 342g/mol
mass of solvent = w1 = 90% of 100 = 90g
m of sucrose = 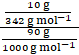
⇒ m = 0.325
now, ∆Tf = Kf![]() m
m
273.15 – 269.15 = Kf![]() 0.325
0.325
Kf = 12.3
In case of glucose, assuming 100g of solution
Mass of solute = 10% of 100 = 10g
Mass of solvent = 90g
Now, m of glucose = ![]() = 0.617
= 0.617
Kf = 12.3 as obtained above
So, ∆Tf = Kf × m
= 12.3 × 0.617 = 7.59
So, freezing point of solution = 273.15 – 7.59
= 265.56K