Write the reactions involved in the following reactions :
(i) Clemmensen reduction
(ii) Cannizzaro reaction
(i) Clemmensen reduction
The carbonyl group of aldehydes and ketones 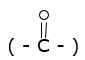 is reduced to CH2 group on treatment with zinc amalgam (Zn-Hg) and concentrated hydrochloric acid (HCl).
is reduced to CH2 group on treatment with zinc amalgam (Zn-Hg) and concentrated hydrochloric acid (HCl).
>C=O ![]() >CH2 + H2O
>CH2 + H2O
(ii) Cannizzaro reaction
α hydrogen is the hydrogen attached to carbon which is immediately next to or attached to carbon of –C=O group in aldehydes.
So, aldehydes without α hydrogen undergo self oxidation and reduction in presence of concentrated alkali to give a molecule of alcohol (reduction) and a carboxylic acid salt (oxidation). For ex-
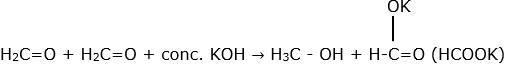
6